Impurity profiling is used to identify and quantify impurities in pharmaceutical products.
The issue of impurities concerns active ingredients and excipients, the drug or the packaging. The undesirable substances can arise during synthesis and production or subsequently form during storage.
The sources of impurities:
- Synthesis: The retention of reagents, solvents or intermediates in the pharmaceutical product
- Degradation Impurities: The formation of undesirable substances due to degradation of the active ingredient
- By-products: Unavoidable by-products from chemical reactions
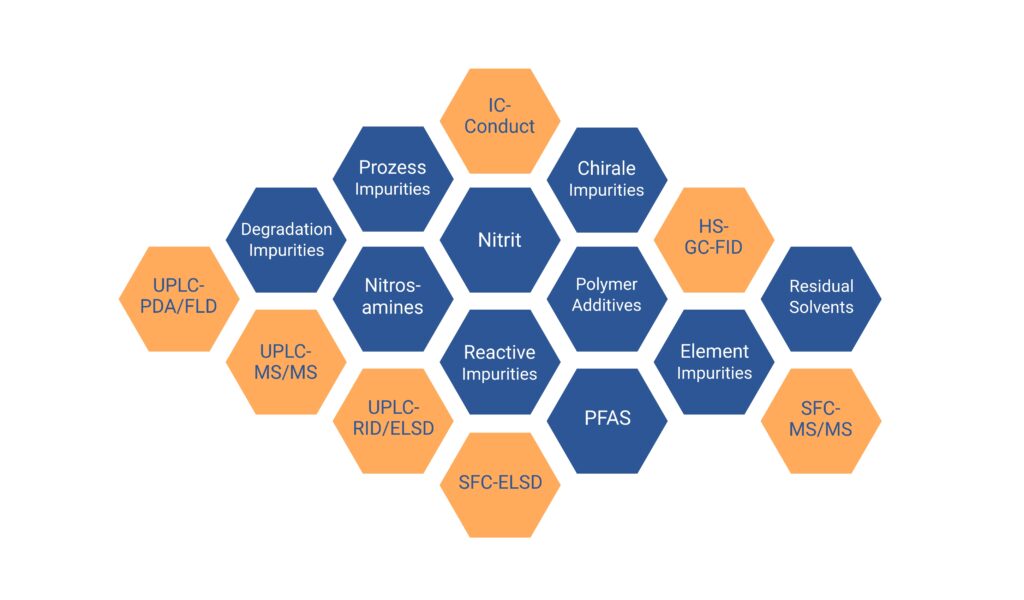
In addition, chiral compounds, nitrosamines, nitrites, reactive impurities such as aldehydes and peroxides, element impurities and extractables & leachables such as polymer additives from packaging are among the impurities.
For identification and quantification, we use state-of-the-art analytical systems such as UPLC-DAD/FLD/RID as well as MS or MS/MS and SFC in conjunction with various detection methods.
Impurity profiling guarantees that the contamination is within a range that has no negative impact on either the efficacy or the safety of the pharmaceutical.
As an analytical and method development laboratory, Chromicent is a driver of innovation in the field of impurity profiling. As a competent partner, we are at your side in a variety of ways – contact us, we will be glad to advise you.